Anti-cancer vaccine
Anti-cancer immune mechanism of the dendritic cells
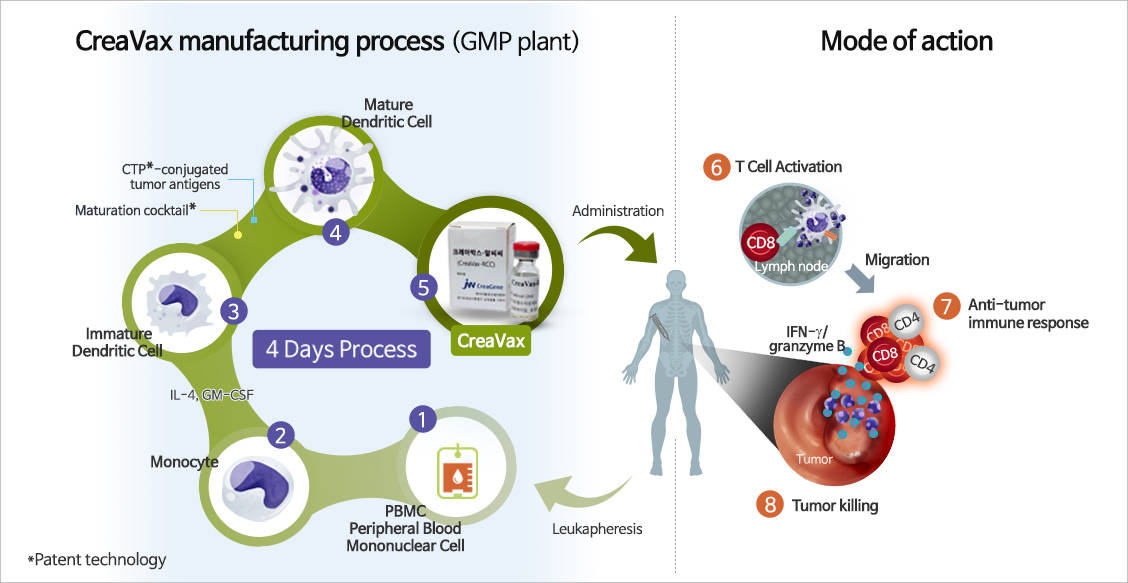
Dendritic cells in the body absorb antigens (virus, bacteria and diseases-specific protein, etc.) and provide information on them to the surface of the cells. This, then, executes the function of combating diseases by inducing the immune reaction of T cells that are specific for the cancer or infectious virus.
Stages of induction of immunity by dendritic cells are as follows:
- Dendritic cells that detected cancer antigens peptide become mature and move to the secondary immune organ by producing a large quantity of homing receptors
- Gathers T cells in inactive state to its surrounding by producing dendritic cell-specific C-C chemokine(DC-CK1)
- Delivers the information on the cancer antigens to the T cells through the interaction between the cancer antigens peptide contained in the dendritic cell MHC and the T cell receptor (TCR)
- Induce proliferation and activation of T cells that are specific for the cancer antigens
Characteristic of dendritic cell vaccine for cancer
- SafetyVery low toxicity and side effects using the autologous cells from patient
- Recurrence prevention effectPrevention of metastasis or recurrence of cancer by inducing the immunological memory on the cancer
- ConvenienceMaintain the quality of life by enabling outpatient treatment
- Potential combination therapyIt can be administered concurrently with chemical therapeutics in order to enhance the treatment effect
- Induction of tumor antigen specific immune responsesSelective killing of cancer cells applying cancer specific antigens
Improvement of antigen presentation ability using CTP technology
Anti-cancer dendritic cell vaccine
- Anti cancer vaccine for liver cancer
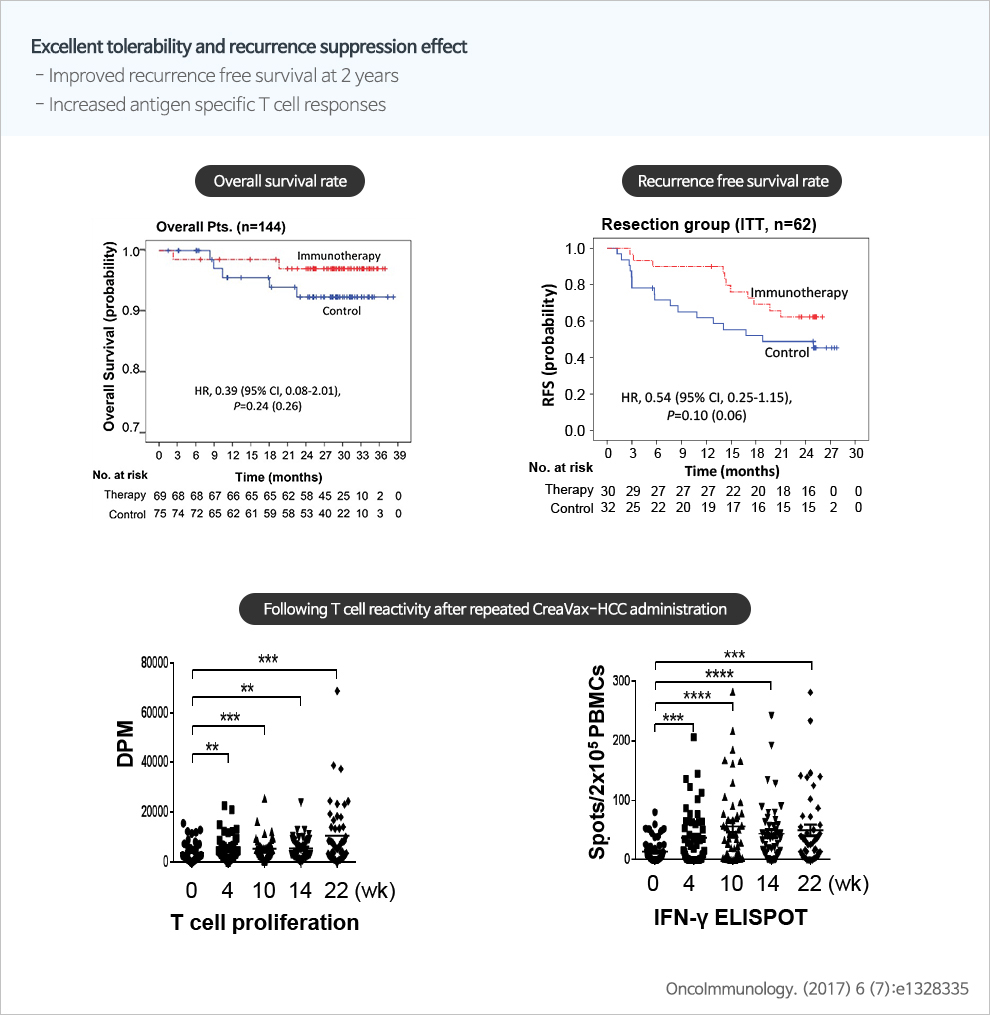
The total number of incidence of liver cancer in Korea in 2015 was 15,757 cases for both men and women, and is ranked the 6th among all the cancers (accounting for 7.3%). It is a cancer with the 2nd highest mortality rate at 15.1% among all the cancers. Hepatocellular carcinoma accounts for approximately 75% of all the liver cancers. Removal of the primary cancer through surgical or non-surgical method is generally performed as the primary treatment. However, it displays very high recurrence rate of 70% in the patients who had undergone surgery within 5 years. The prognosis of the recurred liver cancer is also very adverse in most cases. Therefore, there is a need to develop therapeutics with high medicinal efficacy in inhibition of fundamental recurrence of cancer and in the progressing cancer.
JW CreaGene has selected liver cancer-specific antigens that have high level of manifestation in the liver tissues of Koreans and that has been purified to high level by applying CTP technology, and applied it to the dendritic cell cancer vaccine. It is anticipated that this will activate the anti-cancer actions by inducing liver cancer-specific immune reaction. Phase II of the clinical research for the therapeutics for Hepatocellular carcinoma that JW CreaGene is developing has been completed successfully and Phase III of the manifold clinical research has commenced in order to secure conclusive evidence of effectiveness on the basis of the results. - Anti cancer vaccine for brain cancer
It is difficult to treat malignant neuroglioma (Glioblastoma multiform), which is a type of primary brain cancer, because it grows by infiltrating into the surrounding brain tissues. It is known to be a malignant cancer with highly adverse prognosis of existing treatments including surgery, and anti-cancer radiation and chemical therapies to the extent of limiting the purpose of treatment to slowing down of the infiltration of the cancer tissues. In the case of Glioblastoma multiform, the 1, 2, 3 and 5-year survival rates are 47.2%, 20.0%, 13.0% and 8.9%, respectively, with the median period of survival at approximately 1 year.
JW CreaGene confirmed the anti-cancer effects of the dendritic cell therapeutics through researcher clinical test and phase I/II clinical research is currently conducting to develop the therapeutics for the purpose of inhibiting recurrence and metastasis after the primary treatment with dendritic cells by discovering and sensitizing the stem cell cancer antigens that is known to play central role in recurrence of cancers. - Anticancer vaccine for kidney cancer
Renal cell carcinoma accounts for approximately 3% of the malignant tumor and approximately 88.7% of the cancer occurring in kidney in adults. As of the end of 2015, the total number of manifestation of renal cell carcinoma was 4,555 cases. Approximately 30% of these are discovered in the state of metastasis at the time of detection, and it is a disease with highly adverse prognosis since it progresses into remote metastasis in approximately 20~50% of the patients eventually even for the patients with localized lesion.
In the case of metastasized renal cell carcinoma, the 5-year survival rate is only in the range of 2~5% with the report of the survival period of about 6~10 months. Currently, surgical procedure, chemical anti-cancer therapy and biological therapy, etc. are used to treat renal cancer.
Dendritic cell therapeutics developed by JW CreaGene is used for metastatic renal cell carcinoma patients, for whom nephrectomy can be performed, for the purpose of inhibition of progress into solid tumors. - Anti cancer vaccine for prostate cancer
Prostatic cancer occurs mostly in middle aged men over the age of 45 years. Its prevalence rate is increasing due to the aging of the society and westernization of the dietary habits. Although the incidence rate of prostate cancer is ranked the 7th in Korea, rate of increase in its manifestation is getting higher every year.
The prostate cancer vaccine of JW CreaGene is used for the prostate cancer patients who resist hormone therapy by using highly pure dendritic cells with even properties, which have been differentiated within the monocyte in the blood. Prostate cancer-specific antigen is selected and manufactured in-house, and immune reaction targeted at the prostatic cancer is induced by fusing CTP, which is a drug delivery technology developed by our company. Phases I/IIa of the clinical researches on the anti-cancer vaccine for hormone refractory metastatic prostatic cancer, which is aimed at assessing the safety and exploratory effectiveness, have been completed.
Reference: Data announced by the Korea Central Cancer Registry in 2015
Autoimmune disease Therapeutics
Dendritic cells display different properties depending on the stages of maturation.
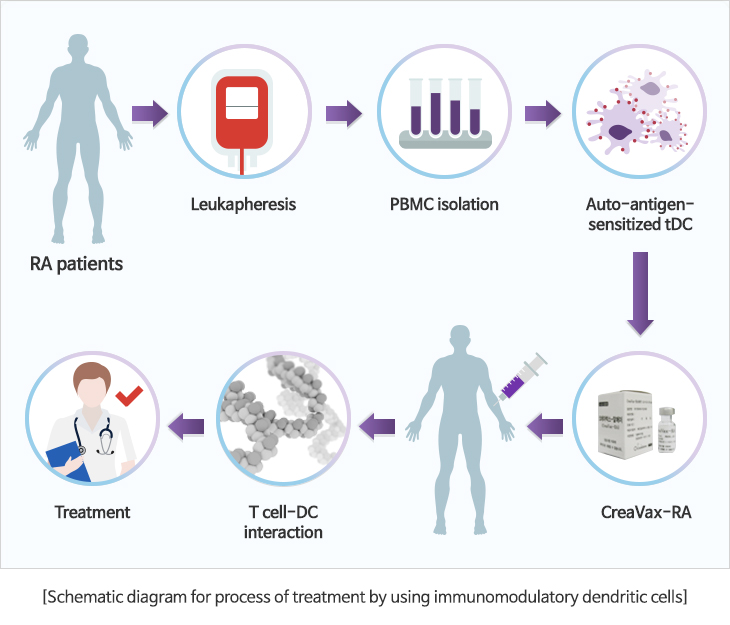
That is, although the mature dendritic cells (mature DC) can be used for anticancer vaccine since it displays immune inducing function, it has been reported that dendritic cells that have not been fully matured (immature DC, semi-mature DC) can be used for treatment of rejection reaction displayed in autoimmune disease such as Type I diabetes, atopy and rheumatic arthritis, and that follows tissue transplant since it has immunomodulatory function.
This is based on the principle that treatment is possible due to the inhibition of the activation and proliferation of the immune cells related to the excessive immune reaction against autologous antigens by the antigens-specific immunomodulatory dendritic cells.
Rheumatic arthritis, which is a representative autoimmune disease, is a disease in which inflammation is manifested and cartilage tissues and bones are damaged due to the gathering of the lymphocytes to the joints in the body due to the abnormal immune reaction in the body. Worldwide, the number of patients is estimated to be approximately 1~2% of the entire population, while it is estimated that the number in Korea is equivalent to approximately 1.4% of the national population (700,000).
JW CreaGene succeeded in confirming the safety and effectiveness through the non-clinical test by manufacturing dendritic cells for which the rheumatic arthritis-specific antigens has been sensitized on the basis of our manufacturing technology for immunomodulatory dendritic cells. Since then, we completed executing clinical tests aimed at confirming the validity and safety of the dendritic cell-based therapeutics for rheumatic arthritis.